Photo credit: Annals of Botany
FIG. 1.
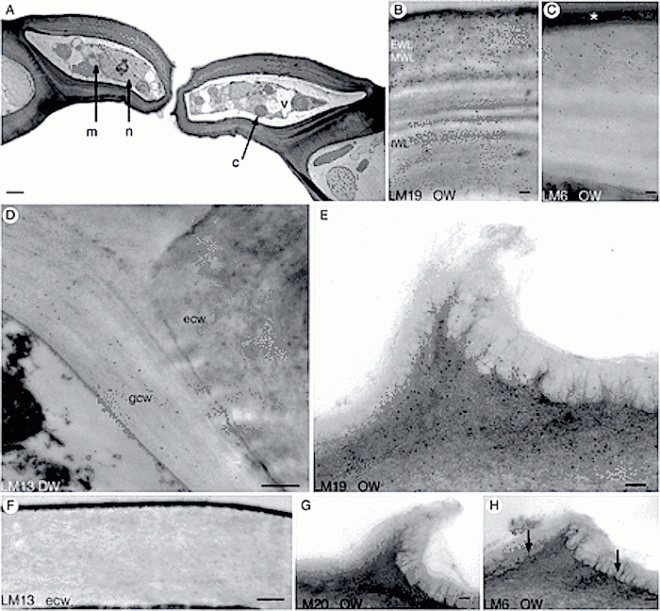
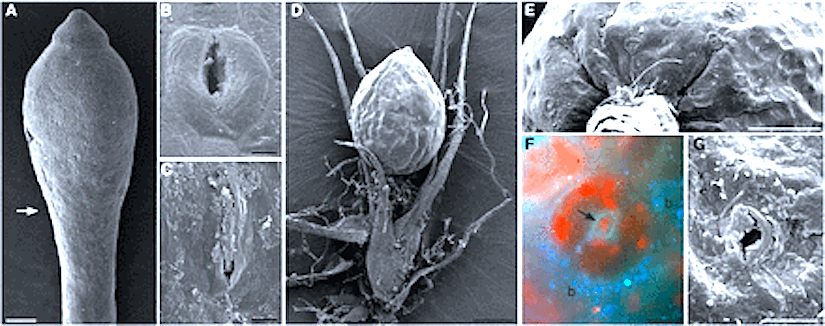
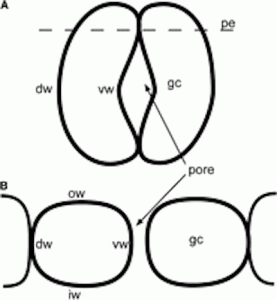
Mature capsules of Funaria. (A) Scanning electron micrograph of expanded capsule with stomata in irregular rows and files on apophysis (arrowheads). (B) Drawing of stomata distribution in the apophysis of mature capsule. (C, D) Scanning electron micrographs of spongy tissue inside the capsule. (E) Scanning electron micrograph of apophysis showing slightly raised stomata covered by smooth cuticle that is thickened around the pore (arrow). Scale bars: (A, C) = 500 µm; (B) = 35 µm; (D) = 100 µm; (E) = 10 µm.
Patterning of stomata in the moss Funaria: a simple way to space guard cells
by Merced A.,
Renzaglia K. S.
(2016)
in Ann Bot (2016) – 117(6):mcw029 · April 2016
doi: 10.1093/aob/mcw029 – First published online: April 23, 2016
Abstract
Background and Aims
Studies on stomatal development and the molecular mechanisms controlling patterning have provided new insights into cell signalling, cell fate determination and the evolution of these processes in plants. To fill a major gap in knowledge of stomatal patterning, this study describes the pattern of cell divisions that give rise to stomata and the underlying anatomical changes that occur during sporophyte development in the moss Funaria.
Methods
Developing sporophytes at different stages were examined using light, fluorescence and electron microscopy; immunogold labelling was used to investigate the presence of pectin in the newly formed cavities.
Key Results
Substomatal cavities are liquid-filled when formed and drying of spaces is synchronous with pore opening and capsule expansion. Stomata in mosses do not develop from a self-generating meristemoid as in Arabidopsis, but instead they originate from a protodermal cell that differentiates directly into a guard mother cell. Epidermal cells develop from protodermal or other epidermal cells, i.e. there are no stomatal lineage ground cells.
Conclusions
Development of stomata in moss occurs by differentiation of guard mother cells arranged in files and spaced away from each other, and epidermal cells that continue to divide after stomata are formed. This research provides evidence for a less elaborated but effective mechanism for stomata spacing in plants, and we hypothesize that this operates by using some of the same core molecular signalling mechanism as angiosperms.
=================
Ann Bot-2016-ContentSnapshoot
AnnalsofBotany
Volume 117 Number 6 2016









You must be logged in to post a comment.