Foliar epidermal studies in Amaranthaceae
by Barber S., Dhingra H. R. (1985)

in Current Science 54(14): 707-709 –
https://www.jstor.org/stable/24088632?seq=1#page_scan_tab_contents


by Barber S., Dhingra H. R. (1985)

in Current Science 54(14): 707-709 –
https://www.jstor.org/stable/24088632?seq=1#page_scan_tab_contents



by El-Ghamery A. A., Sadek A. M., Abdelbar O. H. (2017)
Abbas A. El-Ghamery, a Ahmed M. Sadek, a Ola H. Abdelbar, b
===
in Annals of Agricultural Sciences 62(1): 1-9 – https://doi.org/10.1016/j.aoas.2016.11.001 –
https://www.sciencedirect.com/science/article/pii/S0570178316300331
Abstract
A study of anatomical features of mature leaves and stems (at fruiting stage) of 12 Amaranthus taxa (Family: Amaranthaceae) shows high variation between them and supplied new characters.
The internal structures were evaluated to clarify their effectiveness in solving taxonomic complexity and identification difficulty in this genus. Observation of the transections of blades showed that the epidermis is uniseriate, ground tissue consists of angular collenchyma and thin parenchyma. The vascular bundles shape has three patterns crescent, ring, ovate. Also they may be united or separated while the midrib shape in cross section has two patterns in which U-shaped, cordate or crescent bundle occurs. All leaves are petiolate.
The examination of the petioles exhibits new and varied characters such as petiole shape (cross section), vascular bundles (shape, number, arrangement).
While the resulted characters from the observation of the stem structure showed less variation. Nineteen qualitative characters with 38 character states resulted from leaf anatomy. Only (8) characters were sufficient to generate an identification anatomical key. DELTA program was used in key-generation.
Also different measurements were carried out by a photo analysis program (Image J), such as lamina thickness, mesophyll thickness, area of upper and lower epidermal cells and thickness of upper and lower epidermal cells to exhibit most possible dissimilarities between the studied species.
Type of stomata anomocytic only in all species except species (7) it is anomocytic and hemiparacytic.

by Konarska A. (2017)
===
in Protoplasma. 254(1): 523–537 – doi: 10.1007/s00709-016-0972-0 –
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5216110/
Abstract
In entomogamous plants, the presence and function of floral secretory structures, whose main role is to attract pollinators, is strictly associated with the pollination ecology and hence the reproductive success of the plant.
The aims of the present paper were to analyse the micromorphology and anatomy of flower nectaries and stigmas in Viburnum opulus and V. lantana and to determine the function and microstructure of inflorescence trichomes in both taxa using light and scanning electron microscopy as well as histochemical assays. It was found that stigmas were formed by papillae, which contained lipids, polysaccharides, tannins, and pigments. Stigmatic secretion proceeded via cuticular pores. Floral nectaries formed a thick layer around the styles, and nectar was secreted through numerous nectarostomata. There were no traces of vascular bundles penetrating the nectary tissue. In turn, numerous tannin deposits were observed in the cells of the glandular parenchyma. Pedicels, hypanthia, and bracts had mainly peltate and capitate glandular trichomes as well as stellate non-glandular trichomes (in V. lantana). The trichomes were shown to contain lipids, mucilage, and tannins. Many similarities in the flower and nectaries microstructure and considerable heterogeneity were observed in the examined Viburnum species. Knowledge of the microstructural characteristics of flowers, nectaries, and trichomes may be important for the phylogenesis and taxonomy of the genus Viburnum and the family Adoxaceae. Additionally, floral and nectaries features are helpful in assessment of the relatedness between taxa and provide better understanding of the floral biology and pollination ecology.
Between the epidermal cells, there were densely arranged anomocytic nectarostomata (Table (Table2,2, Fig. 3c–e). Open stomata with a visible secretion in their pores or numerous granules on the surface were observed most frequently (Fig. 3d, e). The stomata on the convex part of the gland were arranged chaotically, whereas the direction of the long axis of the stomata located on the hypanthium-bordering part was most often consistent with the vertical axis of the style (Fig. 3c).

by Ghazalli M. N., Talip N., Latiff A., Masrom H., Salmaniza S., Nurshahidad M. R. (2018)
MOHD. NORFAIZAL, G.1,2*, NORAINI, T.2, A. LATIFF2, H. MASROM1, SALMANIZA, S. 1 AND M. R. NURSHAHIDAH1
1 Programme of Genetic Resources and Germplasm Conservation Management, Centre of Genebank & Seeds, MARDI Headquarters, Persiaran MAEPS-MARDI, 43400 Serdang, Selangor
2 School of Environmental and Natural Resources Science, Faculty of Science and Technology, Universiti Kebangsaan Malaysia, 43600 Bangi, Selangor
===
in Asian Jr. of Microbiol. Biotech. Env. Sc. 20 (2): 387-403 –
ISSN-0972-3005 –
AJM-62.pdf
Abstract –
Sapindaceae is one of the main angiosperm plant families and this family includes 150 genera with ca. 2000 taxa that widespread in the tropical and subtropical regions of the world.
A study on the variation of stomatal types and anticlinal wall patterns was undertaken on 43 taxa belonging to 19 genera of Malaysian Sapindaceae.
Results have shown that there were four types of stomata present and four types of anticlinal wall patterns exist in Malaysian Sapindaceae taxa. Stomata types consist of tetracytic, paracytic, staurocytic and anomocytic stomata.
Whilst for the anticlinal wall patterns vary from sinuous, wavy, slightly curvy and straight patterns. A majority of species were having homostomatic and hipostomatic stomata, except for Lepisanthes amoena, Mischocarpus sundaicus, Nephelium cuspidatum var. robustum, Nephelium cuspidatum var. eriopetalum and Trigonachras acuta which have heterostomatic stomata feature.
Stomatal index (SI) ranging between 2.5% – Litchi chinensis and the highest found in Dodonaea viscosa with SI 25.98%.
The combination of stomatal types, anticlinal wall patterns and also in the stomatal index proved to have taxonomic value especially in assisting species identification for Malaysian Sapindaceae members.
by Przywara L., Pandey K.K., Sanders P. M. (1988)
===
in New Zealand Journal of Botany 26(2):179-182- DOI: 10.1080/0028825X.1988.10410110 –

by Huma Z. E., Hameed I., Sher U., Chen J.-M., Wang Q.-F. (2018)
1 Zill-E-Huma, 2 Ishfaq Hameed, 2 Uzma Sher, 3 Jin-Ming Chen and 3 Qing-Feng Wang
1 Department of Botany, University of Peshawar, Pakistan
2 Department of Botany, University of Chitral, Pkistan
3 Key Laboratory of Aquatic Botany and Watershed Ecology, Wuhan Botanical Garden, Chinese Academy of Sciences, Wuhan 430074, China
===
in Scholarly Journal of Agricultural Science 8(1):. 1-6 – ISSN 2276-7118 –
Click to access Huma%20et%20al.pdf
Abstract
Stomata are consequential as gaseous and dihydrogen monoxide exchange organs for most of the plants and are proximately cognate to photosynthesis, respiration and transpiration.
In this study, we compared the stomatal characteristics of epidermis of leaves of Brassica campestris and B. napus, two economically consequential plant species in Brassica (Brassicaceae).
Leaves of both species are dorsoventral.
Types of stomata observed on upper surface of B. campestris leaf were diacytic, anisocytic, holoparacytic, hemiparacytic and anomocytic and on lower epidermis were hemiparacytic, anomocytic and anisocytic stomata.
Stomatal types observed on upper epidermis of B. napus were anomocytic, desmocytic, hemiparacytic and anisocytic and on lower epidermis of B. napus were anomocytic anisocytic and diacytic.
In B. campestris the highest stomatal density was recorded on the adaxial side while in case of B. napus it was highest on the abaxial surface.
Percentage of closed stomata was more on the abaxial side of both species as compared to the adaxial side.
There were diminutive distinctions between mean length and width of the stomatal pores and sentinel cells of the adaxial and abaxial surfaces of the both plant species.
There was a positive paramount cognation between the epidermis and stomata of the same leaf surface in both plant species.
It is concluded from the present study stomata on the leaves surfaces are bio-designators. Their numbers, arrangement, types, distribution, indices, densities and frequencies pellucidly show the type of the environment in which the study plants grow.
Mendes de Gouveia N. A. (2017)
Nelson Alexander Mendes de Gouveia,
===
in Thesis MAGISTER SCIENTIAE In the Faculty of Natural and Agricultural Sciences Department of Plant Sciences (Botany) University of the Free State –
http://scholar.ufs.ac.za:8080/xmlui/bitstream/handle/11660/6459/DeGouveiaNAM.pdf?sequence=1
SUMMARY
The purpose of this study is to provide an updated taxonomic revision and a molecular phylogenetic investigation of Crabbea Harv. (Acanthaceae) in southern Africa. The taxonomic component of this study entailed a detailed analysis of anatomical, macromorphological and micromorphological data and appropriate descriptions.
Updated distribution maps of each southern African Crabbea species is presented and detailed habitat and ecological information is also provided. Four different identification keys were constructed using leaf anatomy, leaf micromorphology, pollen micromorphology and macromorphology. Type literature, type material and nomenclature for each investigated Crabbea species is critically reviewed. In cases where holotype material could not be located and/or identified, appropriate isotypes, lectotypes, syntypes and/or neotypes were assigned and/or confirmed. Additional herbarium specimens, on loan and electronic scans, from various European and South African herbaria were studied to construct identification keys, species descriptions, distribution maps and obtain ecological and habitat information.
Fresh material was collected for each investigated species. The investigated Crabbea species are all small to medium-sized herbs with cymose inflorescences and corolla being two-lipped, zygomorphic, funnel-shaped with paired, raised bosses. The corolla tube is largely creamish-white but light pink corolla tubes are occasionally found. Growth form, root appearance, stem orientation, position, texture and leaf shape and indumentum are important for species-level identification.
Leaf micromorphological characters are both significant on species level. The occurrence of both amphistomatic and hypostomatic leaves among the investigated species are characteristic of Acanthaceae and could be effectively used to distinguish the investigated Crabbea species from each other. This study provides a first detailed analysis of Crabbea cystoliths. Cystolith attachment width on the adaxial leaf surface proves to be the best character state to split the southern African Crabbea into two groups. The groupings obtained were similar to that of the leaf micromorphology groupings. xxiii Pollen micromorphology divided Crabbea into two groups based on the absence or presence of murus and lumin. However, this character set yielded a different grouping from the leaf micromorphology and anatomy character sets. Pollen grain morphology for certain Crabbea species either remained constant over a geographic range, or varied between and within populations. Macromorphology could key-out all species, except C. cirsioides and C. nana. This character set displays a similar grouping to that of the pollen micromorphology character set. The molecular phylogenetic component of this study resulted in the first molecular investigation of the phylogeny for the southern African Crabbea species. The phylogeny is primarily based on the two chloroplast DNA sequences trnL-trnF and rps16; however, anatomical and morphological characters are also included in the phylogeny to increase the resolution of the tree in absence of the ITS sequences. Molecular phylogenetic results suggest that C. velutina is the first diverging southern African Crabbea species, from the larger Crabbea clade, consisting of C. acaulis, C. angustifolia, C. cirsioides, C. galpinii, C. ovalifolia and C. pedunculata. Within the larger Crabbea clade, C. acaulis forms a distinct clade as well as C. galpinii and C. pedunculata. The molecular results confirm the close relationship between C. galpinii and C. pedunculata. Moreover, within the larger Crabbea clade, molecular data could not clearly resolve and group the sprawling Crabbea species into distinct clades, as in the case of C. angustifolia, C. cirsioides and C. ovalifolia. The end result of this systematic study provides a new insight into the classification of the southern African Crabbea species and the genus Crabbea. Crabbea galpinii and C. pedunculata are confirmed as two separate, sister species and C. nana is now regarded as a synonym of C. cirsioides. Seven Crabbea species are recognised in southern Africa.
by Boso S., Gago P., Alonso-Villaverde V., Santiago J. L., Martinez M. C. (2016)
S. Boso, P. Gago, V. Alonso-Villaverde, J. L. Santiago, M. C. Martinez,
Misión Biológica de Galicia, Consejo Superior de Investigaciones Científicas (CSIC), Salcedo, Pontevedra, Spain
===
in Vitis 55: 17–22 – DOI: http://dx.doi.org/10.5073/vitis.2016.55.17-22 –
4678-Article Text-23136-1-10-20160119.pdf
Summary
A number of studies have highlighted differences in the density of stomata between Vitis species, but few have examined differences between varieties of V. vinifera.
The density and size of the stomata in the lower epidermis of leaves belonging to 12 grapevine varieties, a direct producer hybrid (DPH) involving a V. vinifera and a non-vinifera parent, and the non-vini- fera rootstocks ‘SO4’ and ‘110-Richter’, were therefore examined.
Transparent nail polish peel prints of the area between the main and right lateral veins were pro- duced for 10 leaves per variety. These prints were then examined under a light microscope and the number of stomata in a unit area of 0.196 mm2 counted.
Image analysis software was then used to measure the length and width of all those counted. Rootstock ‘SO4’, ‘Chas- selas Dorée’, ‘Albariño’ and ‘Cabernet Sauvignon’ had the highest stomatal densities (all > 34 stomata per unit area), while ‘Castañal’, ‘Torrontés’ and ‘Caiño Blan- co’ and ‘Jacquez’ (DPH), had the smallest (all < 26.50 stomata per unit area). ‘
Treixadura’ and ‘Caiño Blan- co’ had significantly longer and wider stomata than all the other varieties examined; the DPH ‘Jacquez’ had among the shortest and narrowest.
No relationship was seen, however, between mean varietal leaf size and the stomatal density or stomatal size; nor was any seen between the variables examined and the condition of be- longing to V. vinifera or not.

by Ingole S. N. (2013)
Ingole Shubhangi N.,
Department of Botany, Bar. R.D.I.K. and N.K.D. College Badnera-Amravati, Maharashtra (India)
===
in Int. Res. J. of Science & Engineering 1(3): 85-89 – ISSN: 2322-0015 –
http://oaji.net/articles/2014/731-1396633944.pdf
ABSTRACT
Gmelina arborea Roxb. is a medium sized, unarmed, deciduous tree with whitish gray smooth bark. The plant is also important medicinal. Its flowers are showy, 2.5–3.5 cm long, brownish-yellow usually in small, opposite decussately arranged small cymes of about three flowers along the axis of a densely fulvous-hairy terminal panicles. Floral epidermal features including nature of epidermal cells, stomata and trichomes along with their dimensions of all floral parts are studied.
Epidermal cells are found straight walled in peduncle, bract, stamen, carpel and fruit with cuticular striations in staminal cells, broadly sinuate in calyx, broadly and deeply sinuate in adaxial and abaxial surfaces of corolla lobes respectively.
Peduncle, fruit are astomatic and bracts, calyx and corolla lobes are hypostomatic. They are anomocytic in bract, anomocytic, diacytic and paracytic in calyx and anomocytic and very occasional abaxially on corolla lobe.
Trichomes are of two types non-glandular and glandular, which vary, in minute details on different parts. Unique multiseriate glandular trichomes are exclusively found on filaments.
Floral trichomes are found species specific and suggestive of their functional significance.

by Ingole S. N. (2016)
Shubhangi Ingole,
Department of Botany, Bar. R.D.I.K. and N.K.D. College Badnera, Amravati, Maharashtra, India
===
in WSN 42 (2016) 197-227 – EISSN 2392-2192 –
http://www.worldscientificnews.com/wp-content/uploads/2015/10/WSN-42-2016-197-227.pdf
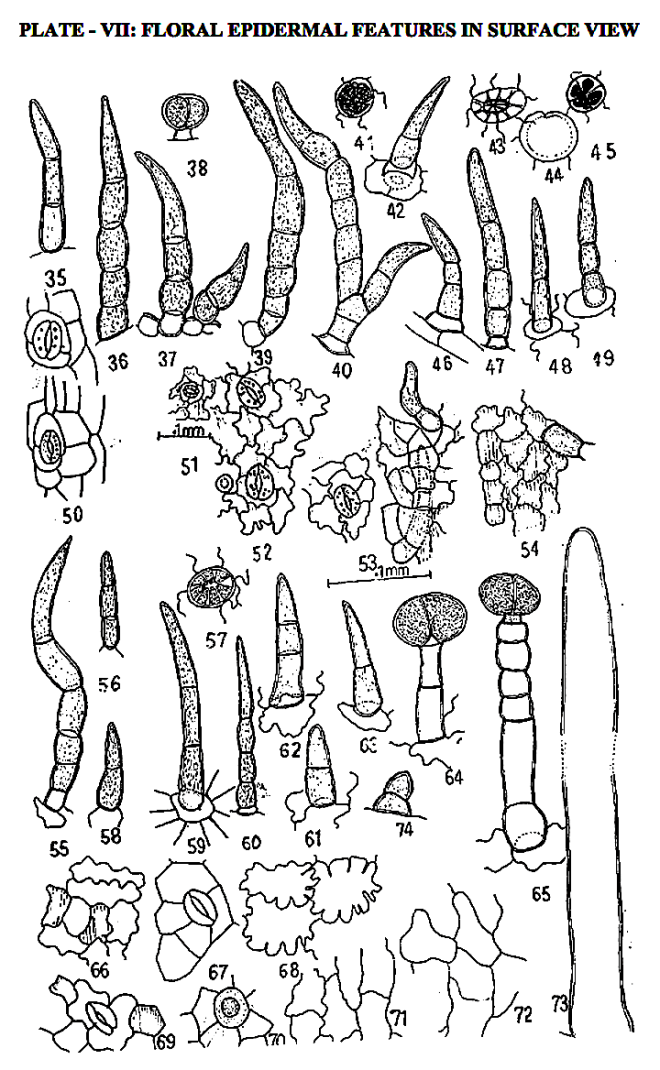
ABSTRACT
The history of herbal medicines is as old as human civilization. In recent times there has been a global trend towards herbal medicines. Many of the botanical, chemical techniques employed in pharmacognosy, quality control of crude drug and its pharmaceuticals can be attempted by different methods of evaluation and one of them is morphological and microscopical studies of crude drug.

In present study pharmacognostical characterization of Clerodendrum serratum (L.) Moon. (Verbenaceae) is attempted which is a medicinal shrub with delightful blooms useful for broad array of ailments from fever to snake bite.
The study includes microscopic study of young stem, vessel elements, petiole-both qualitative and quantitative characters, leaf architecture, leaf constants like stomatal frequency, index number of epidermal cells, their types, vegetative and floral trichomes and epidermal features as anatomical characters are fixed and hence reliable.
You must be logged in to post a comment.