On the functioning of stomates in Funaria.
by Garner D., Paolillo D. J. (1973)
in Bryologist, 76: 423–427. – DOI: 10.2307/3241726 –
http://www.jstor.org/stable/3241726?origin=crossref&seq=1#page_scan_tab_contents
Abstract
by Garner D., Paolillo D. J. (1973)
in Bryologist, 76: 423–427. – DOI: 10.2307/3241726 –
http://www.jstor.org/stable/3241726?origin=crossref&seq=1#page_scan_tab_contents
Abstract
Photo credit: Google
Bryum coronatum
by Egunyomi A. (1982)
in Lindbergia 1982, 8: 121-124. –
http://www.jstor.org/stable/20149430?seq=1#page_scan_tab_contents
Abstract
by Merced-Alejandro A. (2016)

in Open SIUC – DISSERTATIONS > 1038
http://opensiuc.lib.siu.edu/dissertations/1038/
Abstract
As one of the first land plant groups to diversify, mosses are central in understanding the origin, diversification, and early function of stomata. Unlike tracheophytes that have stomata on anatomically complex leaves and stems, mosses bear stomata exclusively on spore-bearing organs (capsules). However, stomata do not occur in all mosses and, indeed, are absence in the earliest-divergent mosses (Takakia, Andreaea, Andreaeobryum and Sphagnum), suggesting that stomata originated in mosses independently of other plants. The occurrence of structurally unique pseudostomata in Sphagnum further confounds the resolution of homology of moss stomata with those of other plants. The five studies included in this dissertation are aimed at clarifying the structure, development and evolution of moss stomata. The first study focuses on the sporophyte anatomy and stomatal ultrastructure in two structurally and phylogenetically divergent mosses, Oedipodium and Ephemerum. Oedipodium is the sister to peristomate mosses and the first extant moss with true stomata. This monospecific genus has an elaborated capsule with an extended apophysis bearing numerous long-pored stomata. In contrast, Ephemerum nests within the peristomate mosses and has a reduced capsule that lacks an apophysis and has a few round-pored stomata. Ultrastructure of stomata is similar in these two mosses and comparable to that of tracheophytes, except that the stomata of mosses are not as structurally distinct from epidermal cells as are tracheophyte stomata. Anatomical features such as the presence of a cuticle, water-conducting cells, and spongy tissues with large areas for gas exchange are more pronounced in Oedipodium sporophytes and support the role of stomata in gas exchange and water transport during development and maturation. The second study examines changes in pectin composition during development in the model moss Funaria. Stomatal movement in tracheophytes requires guard cell walls to be strong, yet flexible, because they have to undergo reversible deformation to open and close the pore. Pectins are necessary for wall flexibility and proper stomatal functioning in seed plants. In this study of Funaria, immunogold-labeling using five antibodies to pectin epitopes was conducted on guard cell walls during development to relate these features to the limited movement of stomata in moss. Movement of Funaria stomata coincides with capsule expansion when guard cell walls are thin and pectinaceous. Walls dramatically increase in thickness after pore formation and the pectin content significantly decreases in mature guard cell walls, suggesting that a decrease in flexibility is responsible for the inability to open a close previously reported in older moss guard cells. Because this was the first study to demonstrate changes in pectin composition during stomatal development in any plant, a similar study was done on Arabidopsis to identify the main types of pectins in guard cell walls. Localization of pectins in guard cell walls of Arabidopsis is similar to mosses in the stage they can move, with homogeneous walls rich in arabinan pectins that are required for wall flexibility. This study extends knowledge of pectin composition from stomata of the moss Funaria with limited stomatal movement to an angiosperm in which stomatal activity is crucial to the physiological health of the plant. The fourth study describes stomata development and internal changes in sporophyte anatomy that lead to formation of air spaces in the moss Funaria. Developing sporophytes at different stages were examined using light, fluorescence and electron microscopy; immunogold-labeling was used to investigate the presence of pectin in the newly formed cavities. Stomata in mosses do not develop from a self-generating meristemoid like in Arabidopsis, but instead they originate from a protodermal cell that differentiates directly into a guard mother cell. Epidermal cells develop from protodermal or other epidermal cells, i.e., there are no stomatal lineage ground cells. This developmental pattern is congruent with the presence of a gene ortholog of FAMA, but not SPCH and MUTE, in Physcomitrella. The final study in this dissertation focuses on the enigmatic Sphagnum. Although true stomata are absent in early-divergent mosses, Sphagnum has specialized epidermal cells, pseudostomata, that partially separate but do not open to the inside. To further understand the structure, function and evolution of pseudostomata, capsule anatomy and ultrastructure of pseudostomata were detailed. As in moss stomata, pseudostomata wall architecture and behavior facilitate capsule dehydration, shape change, and dehiscence, supporting this common function. Unlike other moss stomata, pseudostomata collapse along their ventral walls and they lack a substomatal cavity. Similarities to true stomata include two modified epidermal cells with specialized cell walls that separate by cuticle deposition and respond to drying. Pseudostomata may be interpreted as modified stomata that suppressed substomatal cavity formation, which in turn eliminated pore development. However, clarification of the homology of pseudostomata and moss stomata will require genomic studies integrated with physiological and structural data. The studies described in this dissertation significantly advance our understanding of moss stomatal development and structure, and provide a comparison point to better evaluate the evolution of stomata. Moss capsule anatomy coupled with the exclusive existence of stomata on capsules supports the concept that stomata in moss are involve in gas exchange but also facilitate drying and dispersal of spores. Changes in wall architecture coupled with a decrease in total pectin explain the inability of mature stomata to move. Development and distribution of stomata in Funaria provides evidence of a direct and less elaborated mechanism for stomatal development than described in Arabidopsis. Resolving relationships among early land plants, especially hornworts and mosses, the only bryophyte groups with stomata, is critical to understanding stomata evolution. Evaluated together, the results of this dissertation are consistent with a single origin of stomata in land plants.
Photo credit: Research Gate
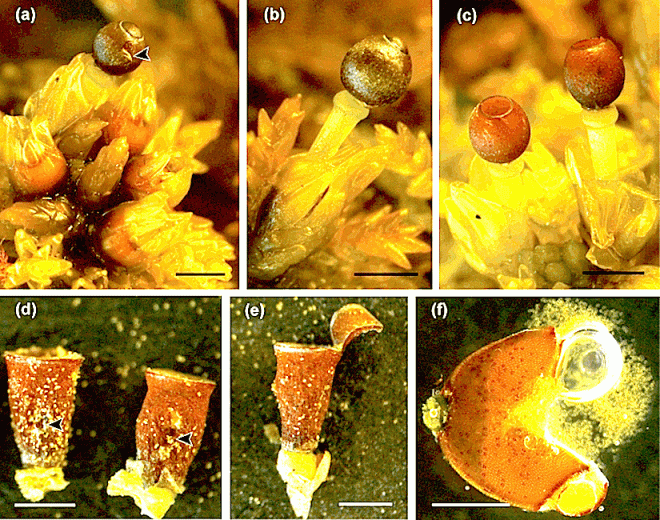
Fig. 1. Sphagnum sporophytes. (A) SEM of close capsule covered by the calyptra (arrowhead) and attached to the pseudopodium (arrow). (B) SEM of pseudostomata in closed capsule. (C) Fluorescence light microscopy of chloroplasts autofl uorescing (arrows) in pseudostoma and epidermal cells. (D) SEM of open capsule. (E) SEM of collapsed pseudostoma in open capsule. (F–I) Light micrographs of semithin cross sections of capsules. (F) Capsule with several layers of capsule wall (arrow) and mature spores. (G) Pseudostoma in close capsule. (H) Pseudostoma in close capsule with ventral walls that begun to separate. (I) Collapse pseudostoma in open capsule. Scale bars: A, D = 1 mm; B, E = 50 μm; C = 10 μm; F = 75 μm; G, H, I = 20 μm.
by Merced A. (2015)
in Am. J. Bot. 102(3): 329, 2015 doi:10.3732/ajb.1400564
Abstract
https://www.researchgate.net/publication/280288657_Highlights_Clues_on_the_evolution_of_stomata_The_enigmatic_pseudostomata_of_Sphagnum [accessed May 6, 2016].
Photo credit: Annals of Botany
Fig. 1.
Diagram of a Funaria stoma, which consists of guard cells with continuous cytoplasm. (A) Transverse section though polar end. (B) Transverse section through pore. DW, dorsal wall; gc, guard cell; IW, inner wall; OW, outer wall; VW, ventral wall.
by Merced A.,
Renzaglia K. S.
(2014)
in Annals of Botany 114:1001-1010. – doi: 10.1093/aob/mcu165 –
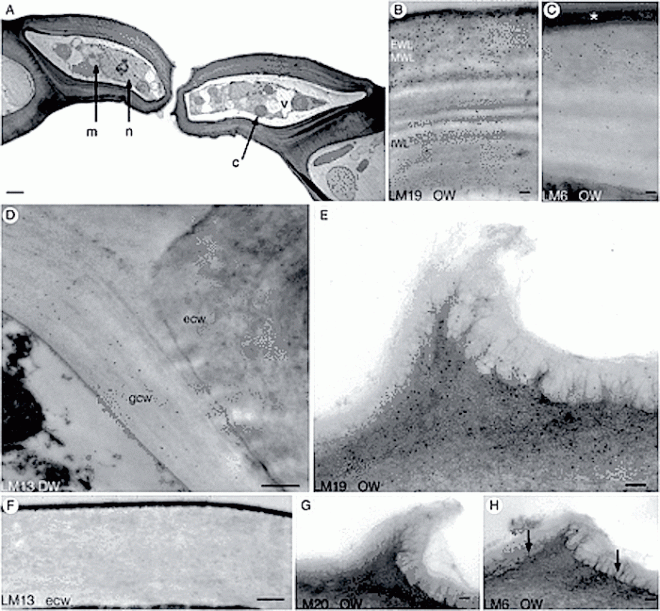
Abstract
Background and Aims
In seed plants, the ability of guard cell walls to move is imparted by pectins. Arabinan rhamnogalacturonan I (RG1) pectins confer flexibility while unesterified homogalacturonan (HG) pectins impart rigidity. Recognized as the first extant plants with stomata, mosses are key to understanding guard cell function and evolution. Moss stomata open and close for only a short period during capsule expansion. This study examines the ultrastructure and pectin composition of guard cell walls during development in Funaria hygrometrica and relates these features to the limited movement of stomata.
Methods
Developing stomata were examined and immunogold-labelled in transmission electron microscopy using monoclonal antibodies to five pectin epitopes: LM19 (unesterified HG), LM20 (esterified HG), LM5 (galactan RG1), LM6 (arabinan RG1) and LM13 (linear arabinan RG1). Labels for pectin type were quantitated and compared across walls and stages on replicated, independent samples.
Key Results
Walls were four times thinner before pore formation than in mature stomata. When stomata opened and closed, guard cell walls were thin and pectinaceous before the striated internal and thickest layer was deposited. Unesterified HG localized strongly in early layers but weakly in the thick internal layer. Labelling was weak for esterified HG, absent for galactan RG1 and strong for arabinan RG1. Linear arabinan RG1 is the only pectin that exclusively labelled guard cell walls. Pectin content decreased but the proportion of HG to arabinans changed only slightly.
Conclusions
This is the first study to demonstrate changes in pectin composition during stomatal development in any plant. Movement of Funaria stomata coincides with capsule expansion before layering of guard cell walls is complete. Changes in wall architecture coupled with a decrease in total pectin may be responsible for the inability of mature stomata to move. Specialization of guard cells in mosses involves the addition of linear arabinans.
Photo credit: Annals of Botany
FIG. 1.
Mature capsules of Funaria. (A) Scanning electron micrograph of expanded capsule with stomata in irregular rows and files on apophysis (arrowheads). (B) Drawing of stomata distribution in the apophysis of mature capsule. (C, D) Scanning electron micrographs of spongy tissue inside the capsule. (E) Scanning electron micrograph of apophysis showing slightly raised stomata covered by smooth cuticle that is thickened around the pore (arrow). Scale bars: (A, C) = 500 µm; (B) = 35 µm; (D) = 100 µm; (E) = 10 µm.
by Merced A.,
Renzaglia K. S.
(2016)
in Ann Bot (2016) – · April 2016
doi: 10.1093/aob/mcw029 – First published online: April 23, 2016
Abstract
Background and Aims
Studies on stomatal development and the molecular mechanisms controlling patterning have provided new insights into cell signalling, cell fate determination and the evolution of these processes in plants. To fill a major gap in knowledge of stomatal patterning, this study describes the pattern of cell divisions that give rise to stomata and the underlying anatomical changes that occur during sporophyte development in the moss Funaria.
Methods
Developing sporophytes at different stages were examined using light, fluorescence and electron microscopy; immunogold labelling was used to investigate the presence of pectin in the newly formed cavities.
Key Results
Substomatal cavities are liquid-filled when formed and drying of spaces is synchronous with pore opening and capsule expansion. Stomata in mosses do not develop from a self-generating meristemoid as in Arabidopsis, but instead they originate from a protodermal cell that differentiates directly into a guard mother cell. Epidermal cells develop from protodermal or other epidermal cells, i.e. there are no stomatal lineage ground cells.
Conclusions
Development of stomata in moss occurs by differentiation of guard mother cells arranged in files and spaced away from each other, and epidermal cells that continue to divide after stomata are formed. This research provides evidence for a less elaborated but effective mechanism for stomata spacing in plants, and we hypothesize that this operates by using some of the same core molecular signalling mechanism as angiosperms.
=================
Ann Bot-2016-ContentSnapshoot. Available from: https://www.researchgate.net/publication/303715177_Ann_Bot-2016-ContentSnapshoot [accessed Jun 2, 2016].
by Duckett J. G., Pressel S., P’ng K. M., Renzaglia K. S. (2009)
School of Biological and Chemical Sciences, Queen Mary University of London, UK.

Pressel S.,
P’ng K. M.,

in New Phytol 2009,183:1053-1063. – doi: 10.1111/j.1469-8137.2009.02905.x.
(PubMed Abstract | Publisher Full Text)
https://www.ncbi.nlm.nih.gov/pubmed/19552695
Read the full article: Wiley Online Library

Stomatal abundance has been widely used to reconstruct palaeo-atmospheres, but there is a view that early-diverging clades of vascular land plants may differ in their responsiveness to atmospheric CO2. Field et al. grow a number of hornworts and moss sporophytes with contrasting stomatal morphologies under different atmospheric CO2 concentrations representing both current and ancient atmospheres, and find that densities and dimensions are mostly unaffected by changes in CO2.
Read the full text: AoB Blog
Photo credit: Am. J. Bot.
(A–C) Oedipodium griffithianum. (A) SEM of capsule and part of apophysis (arrow to bottom of image). Bar = 300 µm. (B) SEM of open long-pored stoma. Bar = 10 µm. (C) SEM of closed pore filled with cuticular waxes. Bar = 10 µm. (D–G)Ephemerum spinulosum. (D) SEM of mature sporophyte attached to gametophyte. Bar = 200 µm. (E) SEM of base of capsule with stomata. Bar = 100 µm. (F) Stoma in fluorescent light treated with DAPI showing blue nuclei and autofluorescing red chloroplasts; the round pore is flanked by wall ledges (arrow), and bacteria are abundant around guard cells (b). Bar = 10 µm. (G) SEM of stoma partially filled with cuticular waxes. Bar = 10 µm.
by Merced A.,
Renzaglia K. S.
(2013)
in American Journal of Botany 100(12): 2318-2327. 2013.
(http://www.amjbot.org/cgi/doi/10.3732/ajb.1300214)

ABSTRACT
• Premise of the study: Mosses are central in understanding the origin, diversification, and early function of stomata in land plants. Oedipodium, the first extant moss with true stomata, has an elaborated capsule with numerous long-pored stomata; in contrast, the reduced and short-lived Ephemerum has few round-pored stomata. Here we present a comparative study of sporophyte anatomy and ultrastructure of stomata in two divergent mosses and its implications for stomata diversity and function.

• Methods: Mature sporophytes of two moss species were studied using light, fluorescence, and scanning and transmission electron microscopy. Immunolocalization of pectin was conducted on Oedipodium using the LM19 antibody.

• Key results: Oedipodium capsules have extensive spongy tissue along the apophysis, whereas those of Ephemerum have minimal substomatal cavities. Stomatal ultrastructure and wall thickenings are highly similar. Sporophytes are covered by a cuticle that is thicker on guard cells and extends along walls surrounding the pore. Epicuticular waxes and pectin clog pores in old capsules.
• Conclusions: Ultrastructure of stomata in these mosses is similar to each other and less variable than that of tracheophytes. Anatomical features such as the presence of a cuticle, water-conducting cells, and spongy tissues with large areas for gas exchange are more pronounced in Oedipodium sporophytes and support the role of stomata in gas exchange and water transport during development and maturation. These features are modified in the reduced sporophytes of Ephemerum. Capsule anatomy coupled with the exclusive existence of stomata on capsules supports the concept that stomata in moss may also facilitate drying and dispersal of spores.
Read the full article: Am. J. Bot.
by Duckett J. G., Pressel S., P’ng K. M., Renzaglia K. S. (2009)
in New Phytol 2009, 183:1053-1063.
(PubMed Abstract | Publisher Full Text)

Summary
Read the full article: Wiley Online Library
You must be logged in to post a comment.