Photo credit: AOB
Drawings of Equisetum stomata. (A) E. palustre; fig. 10 from Duval-Jouve (1864). (B) E. fluviatile; fig. 12 from Riebner (1925).
The remarkable stomata of horsetails (Equisetum): patterning, ultrastructure and development
byErin Cullen,
(2016)
in Ann Bot (2016)doi: 10.1093/aob/mcw094 –
http://aob.oxfordjournals.org/content/early/2016/06/03/aob.mcw094.abstract

Abstract
Background and Aims The stomata of Equisetum – the sole extant representative of an ancient group of land plants – are unique with respect to both structure and development, yet little is known about details of ultrastructure and patterning, and existing accounts of key developmental stages are conflicting.
Methods We used light and electron microscopy to examine mature stomata and stomatal development in Equisetum myriochaetum, and compared them with other land plants, including another putative fern relative, Psilotum. We reviewed published reports of stomatal development to provide a comprehensive discussion of stomata in more distantly related taxa.
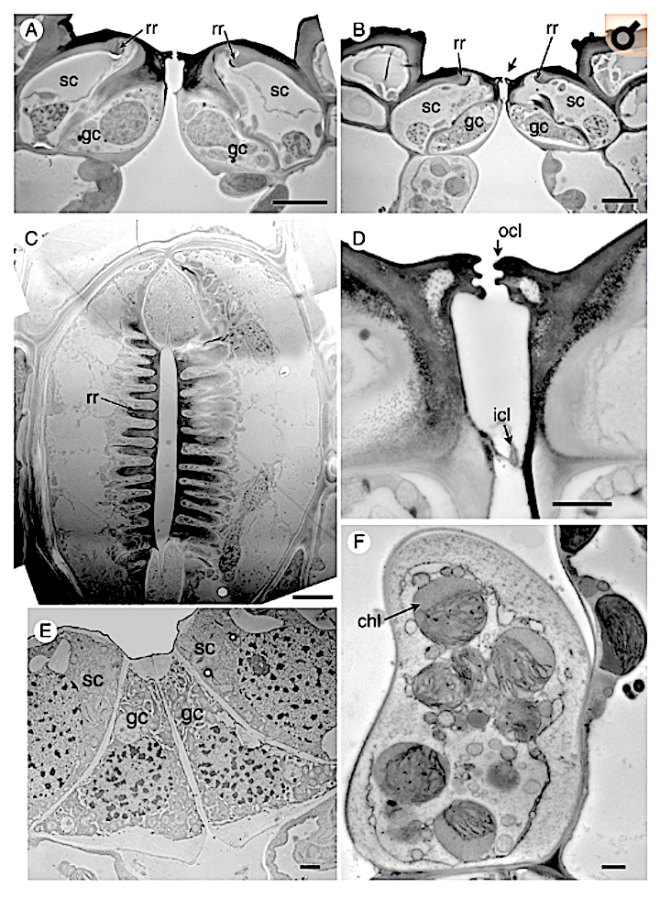
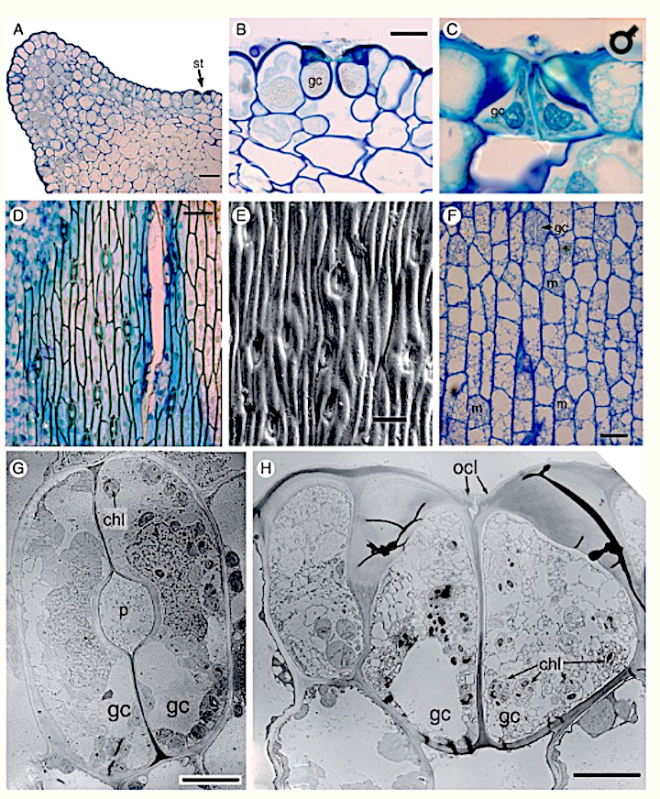
Key Results Stomatal development in Equisetum is basipetal and sequential in strict linear cell files, in contrast with Psilotum, in which stomatal development occurs acropetally. In Equisetum, cell asymmetry occurs in the axial stomatal cell file, resulting in a meristemoidal mother cell that subsequently undergoes two successive asymmetric mitoses. Each stomatal cell complex is formed from a single precursor meristemoid, and consists of four cells: two guard cells and two mesogene subsidiary cells. Late periclinal divisions occur in the developing intervening cells.
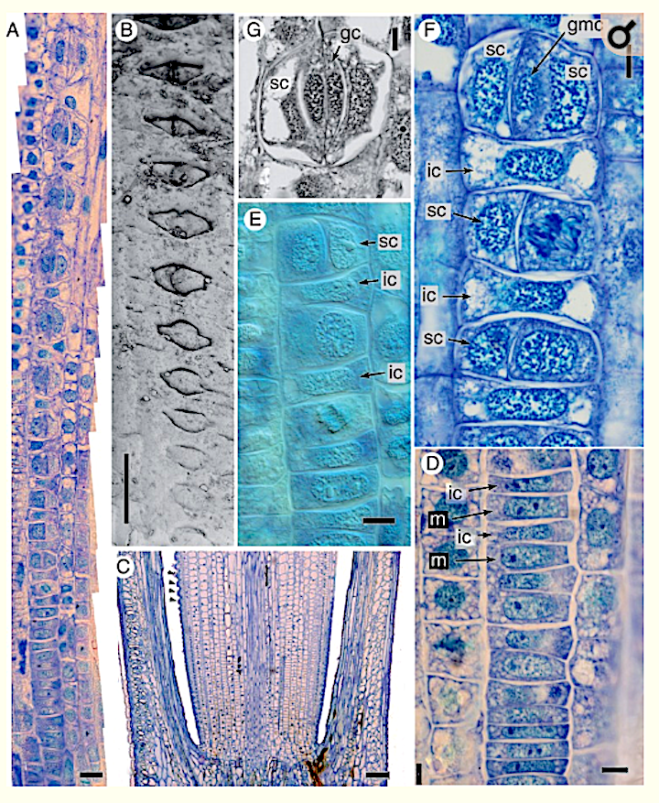
Conclusions In addition to the unique mature structure, several highly unusual developmental features include a well-defined series of asymmetric and symmetric mitoses in Equisetum, which differs markedly from Psilotum and other land plants. The results contribute to our understanding of the diverse patterns of stomatal development in land plants, including contrasting pathways to paracytic stomata. They add to a considerable catalogue of highly unusual traits of horsetails – one of the most evolutionarily isolated land-plant taxa.















You must be logged in to post a comment.