Photo credit: Wiley
(b) Photographs of stomata representative of leaves with maximal and with minimal transpiration. The vitality of the closed guard cell was tested with neutral red. Bar = 25 µm.
Dynamics of ionic activities in the apoplast of the sub-stomatal cavity of intact Vicia faba leaves during stomatal closure evoked by ABA and darkness
, , ,
The Plant Journal, 2000, 24, 3, 297 –
http://onlinelibrary.wiley.com/doi/10.1046/j.1365-313x.2000.00878.x/full
Summary
Stomatal movement is accomplished by changes in the ionic content within guard cells as well as in the cell wall of the surrounding stomatal pore.
In this study, the sub-stomatal apoplastic activities of K+, Cl–, Ca2+ and H+ were continuously monitored by inserting ion-selective micro-electrodes through the open stomata of intact Vicia faba leaves.
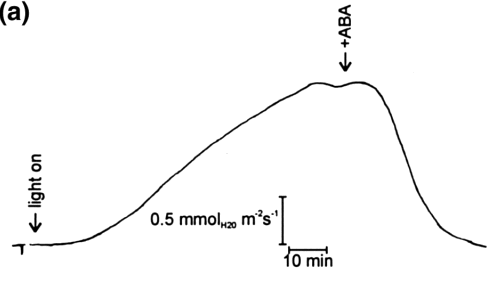
In light-adapted leaves, the mean activities were 2.59 mm (K+), 1.26 mm (Cl–), 64 µm(Ca2+) and 89 µm (H+). Stomatal closure was investigated through exposure to abscisic acid (ABA), sudden darkness or both. Feeding the leaves with ABA through the cut petiole initially resulted in peaks after 9–10 min, in which Ca2+ and H+ activities transiently decreased, and Cl– and K+ activities transiently increased. Thereafter, Ca2+, H+ and Cl– activities completely recovered, while K+ activity approached an elevated level of around 10 mm within 20 min.
Similar responses were observed following sudden darkness, with the difference that Cl– and Ca2+ activities recovered more slowly. Addition of ABA to dark-adapted leaves evoked responses of Cl– and Ca2+ similar to those observed in the light.
K+ activity, starting from its elevated level, responded to ABA with a transient increase peaking around 16 mm, but then returned to its dark level. During stomatal closure, membrane potential changes in mesophyll cells showed no correlation with the K+ kinetics in the sub-stomatal cavity.
We thus conclude that the increase in K+ activity mainly resulted from K+ release by the guard cells, indicating apoplastic compartmentation. Based on the close correlation between Cl– and Ca2+ changes, we suggest that anion channels are activated by a rise in cytosolic free Ca2+, a process which activates depolarization-activated K+ release channels.


You must be logged in to post a comment.