Changes in apoplastic pH and membrane potential in leaves in relation to stomatal responses to CO2, malate, abscisic acid or interruption of water supply.
by Hedrich R., Neimanis S., Savchenko G., Felle H. H., Kaiser W. M., Heber U. (2001)
in Planta 213: 594–601 –
[PubMed]
Abstract
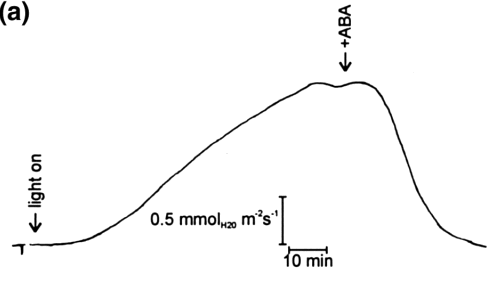
Low CO2 concentrations open CO2-sensitive stomata whereas elevated CO2 levels close them. This CO2 response is maintained in the dark. To elucidate mechanisms underlying the dark CO2 response we introduced pH- and potential-sensitive dyes into the apoplast of leaves. After mounting excised leaves in a gas-exchange chamber, changes in extracellular proton concentration and transmembrane potential differences as well as transpiration and respiration were simultaneously monitored.
Upon an increase in CO2 concentration transient changes in apoplastic pH (occasionally brief acidification, but always followed by alkalinization) and in membrane potential (brief hyperpolarization followed by depolarization) accompanied stomatal closure.
Alkalinization and depolarization were also observed when leaves were challenged with abscisic acid or when water flow was interrupted. During stomatal opening in response to CO2-free air the apoplastic pH increased while the membrane potential initially depolarized before it transiently hyperpolarized.
To examine whether changes in apoplastic malate concentrations represent a closing signal for stomata, malate was fed into the transpiration stream. Although malate caused apoplastic alkalinization and membrane depolarization reminiscent of the effects observed with CO2 and abscisic acid, this dicarboxylate closed the stomata only partially and less effectively than CO2.
Apoplastic alkalinization was also observed and stomata closed partially when KCl was fed to the leaves. Respiration increased on feeding of malate or KCl, or while abscisic acid closed the stomata.
From these results we conclude that CO2 signals modulate the activity of plasma-membrane ion channels and of plasmalemma H+-ATPases during changes in stomatal aperture. Responses to potassium malate and KCl are not restricted to guard cells and neighbouring cells.



You must be logged in to post a comment.