Photosynthesis and leaf anatomy of Anthurium cv. Rubi plantlets cultured in vitro under different silicon (Si) concentrations
by Gomes Dias G. M., Rodrigues Soares J. D., Pasqual M., Lara Silva R. A., de Almeida Rodrigues L. C., Pereira F. J., de Castro E. M. (2014)
Gabrielen de Maria Gomes Dias1* , Joyce Dória Rodrigues Soares1 , Moacir Pasqual1 , Renata Alves Lara Silva1 , Luiz Carlos de Almeida Rodrigues2 , Fabricio José Pereira2Evaristo Mauro de Castro2
1 Federal University of Lavras (UFLA), Departamento of Agriculture, Plant Tissue Culture Laboratory, Postal Office 3037, 37200-000, Lavras- MG, Brazil
2 Federal University of Lavras (UFLA), Departamento of Biology, Postal Office 3037, 37200-000, Lavras- MG, Brazil
===
in Austral. J. Crop Science AJCS 8(8): 1160-1167 – ISSN:1835-2707 –
https://pdfs.semanticscholar.org/8a93/4a54f0acbe7f9c8e0dc43496a05d41d552ed.pdf
Abstract
The silicon can induce beneficial changes in plants, such as the further development of tissues and increased photosynthetic rate. Thus, studies on the anatomical changes resulting from in vitro culture are key to better understanding the development of micropropagated plants.
Therefore, this study was undertaken to evaluate the morphological and physiological differences in plants with the use of silicon added to the medium for the in vitro culture of Anthurium adreaenum cv. Rubi.
Nodal segments of seedlings were established in vitro and inoculated in Pierik medium supplemented with 30 g L-1 sucrose and solidified with 1.8 g L- 1 PhytagelTM. Different concentrations of sodium silicate (Na2SiO3 ) (0.0, 0.5, 1.0 and 2.0 mg L-1 ) were added to the medium. The experimental design was completely randomized with 30 repetitions. The segments were maintained for 100 days in a growth chamber under controlled conditions and evaluated anatomically and scanning electron microscopy (ultrastructurally) and for their photosynthetic capacities.
Medium containing 1.0 mg L-1 sodium silicate promoted the development of higher stomatal densities on the sheets. For the polar (31.38 µm) and equatorial (31.33 µm) diameter of the stomata of the abaxial leaf, the highest averages occurred in the treatment with 2.0 mg L-1 . Greater relative polar and equatorial diameters were estimated with a peak concentration of 1.2 mg L-1 . The increase in the sodium silicate concentration led to thinning of the abaxial and adaxial epidermis.
The thickness of the central rib had a sharp decrease up to 1.3 mg L-1 . For the mesophyll, the control displayed a higher thickness, whereas the addition of sodium silicate to the culture medium promoted a decrease. Seedlings grown in sodium silicate displayed significant differences, with increased photosynthetic and transpiration rates, stomatal conductance and internal CO2 concentrations. As for the ratio between the internal and external concentrations of CO2 , no significant differences were observed. The addition of sodium silicate resulted in increased epicuticular wax deposition and the formation of structures reserved for depositing calcium. Therefore, under in vitro conditions, the addition of sodium silicate to the culture medium affected the photosynthesis and leaf anatomy of A. andraeanum cv. Rubi, developing anatomical and physiological characteristics that contributed to the survival ex vitro.
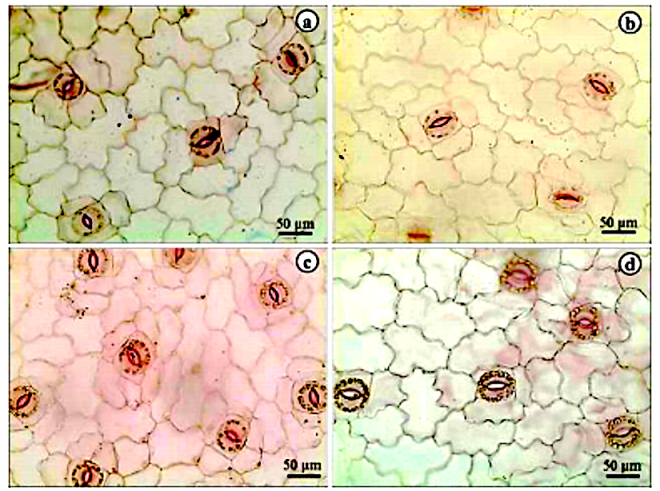
The leaves have stomata flanked on each side by two subsidiary cells parallel to larger stomatal axis (Fig 1A and B). Stomata showing two subsidiary cells displayed parallel to the larger stomatal axis are often classified as type brachyparacytic type (Castro et al., 2009). So, A. andraeanum stomata may be classified as paracytic type. Stomatal types in Araceae are very diverse and some species may show anomocytic, actinocytic, paracytic, and cyclocytic stomata (Wang and Zhao, 2002). Therefore, the brachypracytic stomata described by A. andraeanum may be an important characteristic for correct identification and was also reported by Mantovani et al. (2010) for some Anthurium species. Stomata guard cells showed a large number of chloroplasts and the usual bean-like shape (Fig. 1A). Despite the chloroplasts in stomatal guard cells is a very common anatomical characteristic (Castro et al., 2009) it was not highlighted by previous works in Anthurium anatomy (Wang and Zhao, 2002; Mantovani et al., 2010). Anticlinal cell walls are deeply sinuous in epidermal cells (Fig. 1A-D). Araceae leaves may show straight to very sinuous anticlinal cell walls in epidermal cells but a given species often show just one type (Keating, 2003). In nine Brazilian Anthurium species, Matovani et al. (2010) described from straight to undulated anticlinal cell walls. Therefore, the very sinuous anticlinal cell walls from A. andraeanum may be an important anatomical trait. The stomata are distributed only on the abaxial surface of leaves, classifying the se organs as hypostomatous (Fig 1). Hypostomatous leaves were also described in another Anthurium species by Mantovani et al. (2010) and Saito and Lima (2009).
You must be logged in to post a comment.