Epidermal structure and stomatal types in vegetative and reproductive organs of three species of Bryophyllum
by Jeong W.-G., Kim C. S. (1987)
in Korean J. Bot. 30. (1): 43-58. –
http://agris.fao.org/agris-search/search.do?recordID=KR19890035333
The epidermal structure and stomatal types in vegetative and reproductive organs of three species of Bryophyllum (B. crenatum, B. diagremontian, B. tubiflorum) were described.
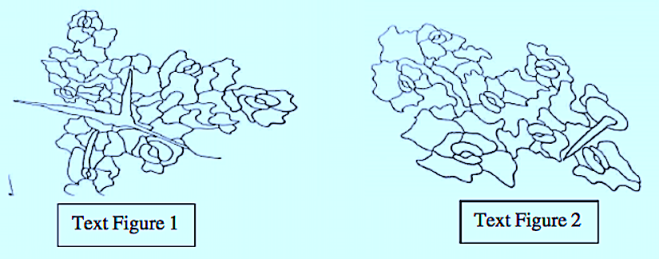
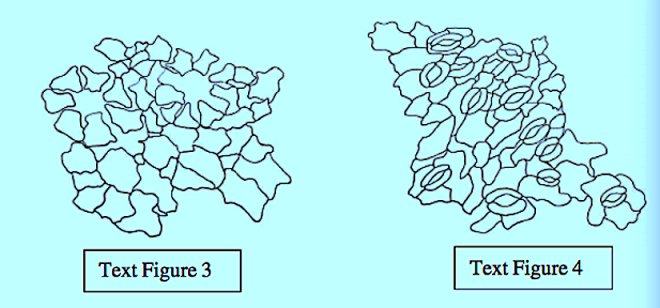
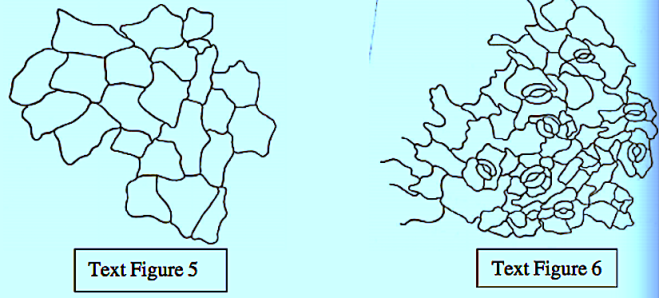
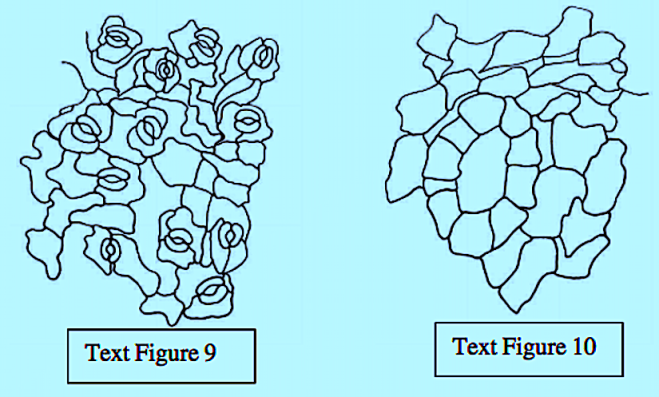
The epidermal cells were polygonal, isodiametric, and rectangular in the leaves and stems, styles, and ovaries. These cells were commonly thick, and arched or sinuous in the leaves, epiphylous buds, petals and ovaries. They were straight in the stems, petioles, pedicels, and peduncles. In both vegetative and reproductive organs, the subsidiary cell walls were commonly thin and mostly arched in all the organs.
The great majority of the mature stomata in all the organs were helicocytic type with a helix of four to six subsidiary cells. The mature stomata varied from organ to organ with regard to the number and arrangement of subsidiary cells.
The ontogenetic type of stomata in all the organs was mostly helico-eumesogenous type. This type was subdivided into three subtypes such as parahelico-eumesogenous, anomohelico-eumesogenous, and diahelico-eumesogenous stomata on the basis of the division angle of the guard mother cell.
Sometimes, the aniso-eumesogenous type was found in various organs. This type was subdivided into three subtypes such as paraniso-eumesogenous, anomoaniso-eumesogenous, and dianiso-eumesogenous stomata.
The tetra-eumesogenous and duplotetra-eumesogenous types were rarely found; the former in the leaf of B. crenatum and the latter in the leaf of B. diagremontiana.
An omomeristic patterns in the mesogenous category of stomatal types was observed in a few organs of all the materials.
A new stomatal type with tetra-eumesogenous stoma within a girdle of three subsidiary cells of aniso-eumesogenous in the leaf of B. diagremontiana was firstly observed in the vascular plants. This stoma was termed the cotetra-aniso-eumesogenous type.
Abnormal stomata such as aborted stomata, single guard cells, stoma with a constricted part in the middle of large guard cells, and arrested stomata were found in the various organs of all the materials.




You must be logged in to post a comment.